No doubt, solar energy is one of the best -if not the best- source of energy we can use to power our homes and increase their value.
It’s clean, efficient, and most importantly, it can save you tons of money each month.
But have you ever wondered how do solar panels work?
Like how can these black or dark blue boards generate electricity?
Well, if you are looking for a super geeky, yet easy-to-understand answer that will make you look smarter at family gatherings, then this article is specially made for you.
In this guide, we will show you exactly how do solar panels work step by step, starting from sunlight hitting the panel to how it’s converted to electricity to power your home.
So without further ado, let’s jump right into what are solar cells made off.
What Are Solar Cells Made Of?
Solar panels are made of semiconductor materials like sand (Si) doped with other elements like Phosphorus (P) and Boron (B) to improve their conductivity and create a P-N junction.
Well, unless you are really good at physics, I bet you didn’t understand a single word of what I just said.
But don’t worry, I’m going to break it all down for you.
What Are Semiconductors?
Semiconductors are materials that have electricity conducting properties that lie between those of an insulator and a conductor.
They can be found in many electronic devices that we use every day, including smartphones, laptops, tablets, and LED lights.
In fact, the semiconductor industry is one of the largest and most important industries in the world!
What makes semiconductors like Silicon such special materials is that they have 4 valence electrons, which means that each Silicon atom can bind with 4 other atoms using covalent bonds.
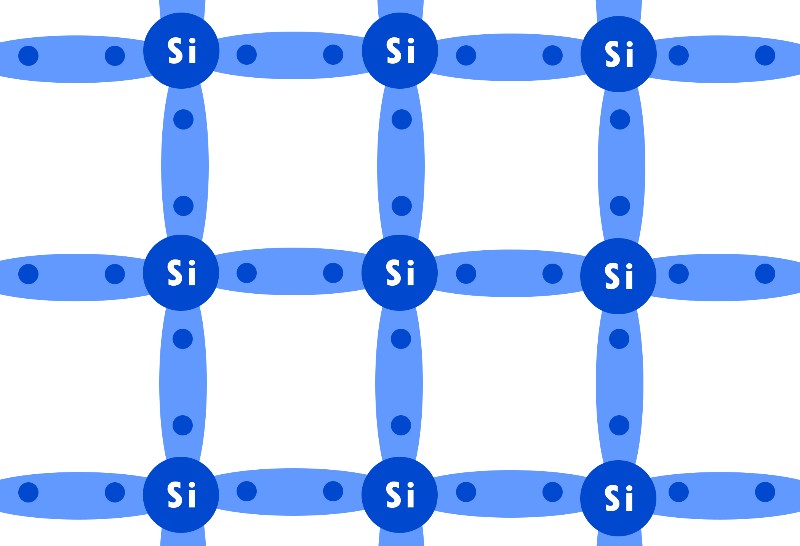
These strong bonds hold the Silicon together and allow for some movement of electrons within the material but they are so strong that electrons can’t break free from their force.
This means that under ideal conditions pure Silicon crystals are considered insulators rather than conductors, as almost all the electrons are used to make bonds and no free electrons are available to conduct electricity.
The words to look for here are ideal conditions and pure Silicon crystals.
As you know, we don’t live in an ideal world, or at least Silicon atoms don’t.
External forces such as heat, pressure, and light can provide some electrons with enough energy to break free from the atom’s covalent bonds, creating a flow of free electrons, which increases the conductivity of the crystal.
This means that pure Silicon crystals can be a conductor or an insulator, depending on the environment.
In the case of pure Silicon crystal, there will be very few free electrons to conduct electricity, as each atom will use its 4 electrons to bind with 4 other atoms, so it can reach equilibrium.
Now, scientists thought of this, how can we increase the number of electrons in each crystal?
And the answer was quite easy. Doping.
Doping is the process of adding impurities to the semiconductor to control the number of free electrons in the crystal.
Depending on the type of impurity, the crystal can be classified as an N-Type or P-Type semiconductor.
What Is An N-Type Semiconductor?
As we mentioned, each Silicon atom has 4 valence electrons and can bind to 4 other atoms.
Now, what do you think would happen if we added an element like Phosphorous (P) that has 5 valence electrons?
Exactly!
Each Phosphorous atom will bind with 4 Silicon atoms, leaving 1 electron to move around freely.
And the more Phosphorous you add, the more free electrons will be available.
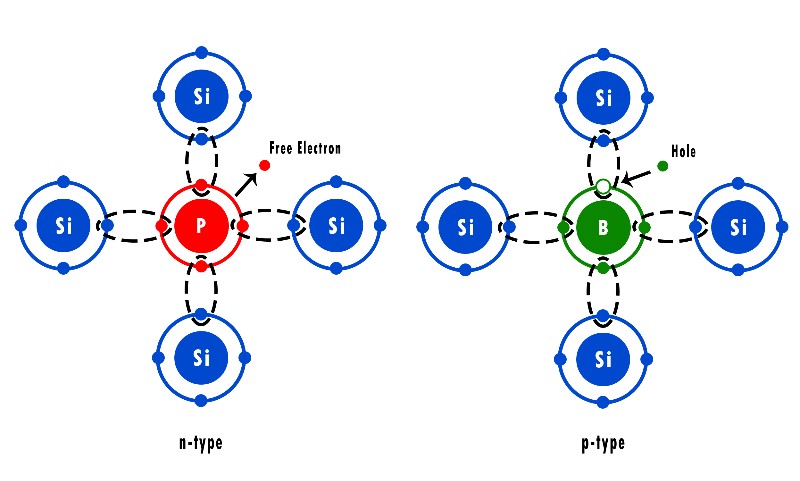
What Is A P-Type Semiconductor?
The difference between N-Type and P-Type semiconductors is that in P-Type, we dope the semiconductor with an element that has 3 valence electrons like Boron.
In this case, each Boron atom will bind with 3 adjacent Silicon atoms and form what we call a “hole“.
In simple terms, a hole is a place in the Silicon crystal where there is a lack of an electron.
And the absence of this electron makes the atom pretty upset, as it really needs this electron to reach equilibrium.
For this reason, this hole becomes a positive charge and it tries its best to fill this gap by attracting electrons from other adjacent atoms, which in turn cause a flow of current AKA electricity.
For a better understanding, think of the Boron atom as a magnet and electrons as small metal coins.
If the magnet is close enough to the coin, it will attract it and the coin will leave a “hole” in its previous position.
This hole will then become a new magnet and will try to attract another coin to reach equilibrium, and so on.
Now, as you know what are P-Type and N-Type semiconductors, it’s time to get into what we call a P-N junction.
What Is A P-N Junction?
As the name implies, this is a combination of an N-Type and a P-Type semiconductor.
The PN junction is made by doping a small region of the semiconductor with N-Type elements and an adjacent region with a P-Type element. The result is what we call the PN junction.
Once you dope the semiconductor, the P-N junction will look something like this:
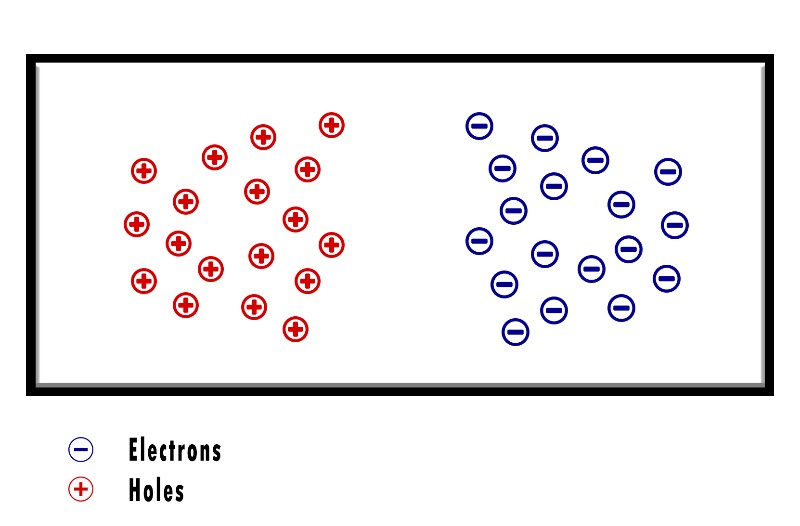
One region will have an excess of electrons (n-region) while the other will have an excess of holes (p-region).
As a result, free electrons from the n-region will travel to the p-region to fill the holes, which creates a negatively charged layer.
And at the same time, the n-region will attract holes, creating a positively charged layer.
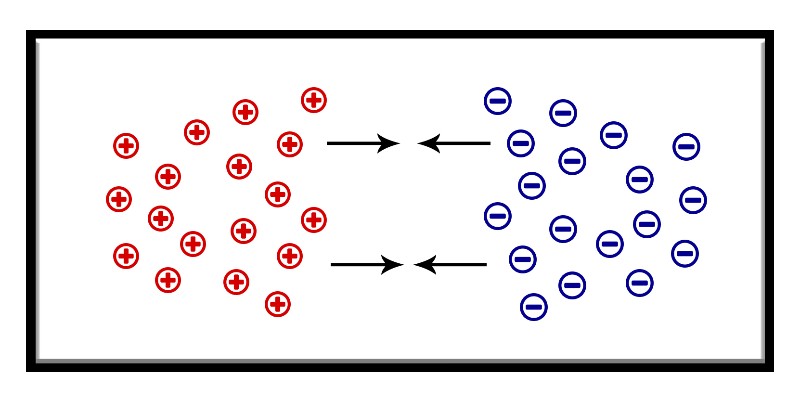
This creates what we call the “depletion region“, which is simply a voltage difference between the 2 regions that resists current from flowing between the two sides of the junction.
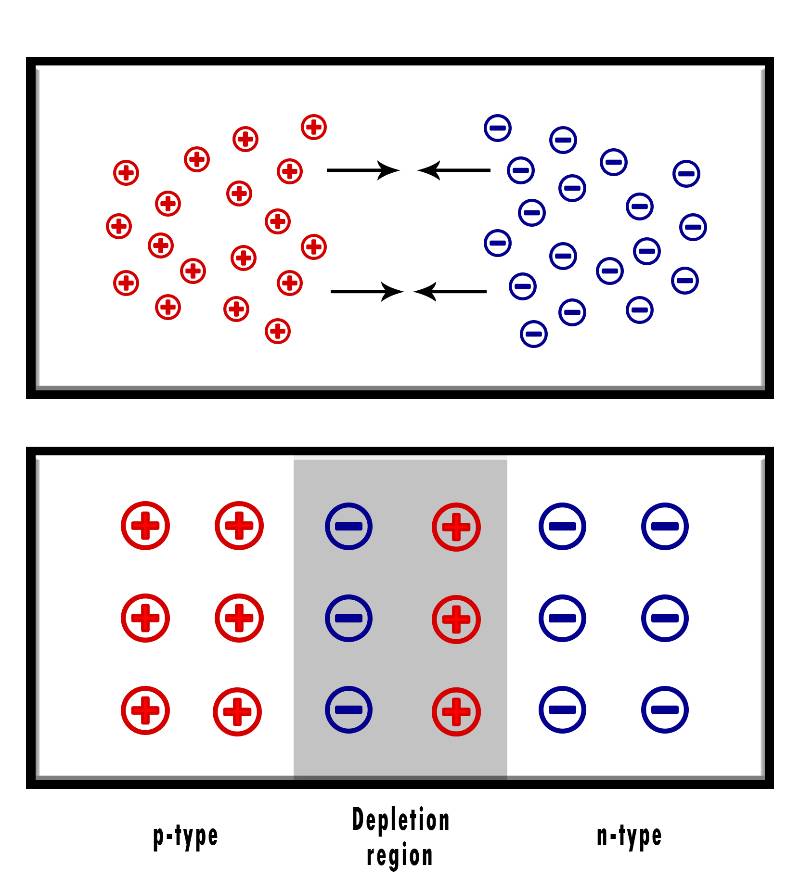
As more and more electrons and holes travel to the opposite side of the junction, the depletion region gets bigger and bigger till the P-N junction reaches equilibrium.
Depletion Region Voltage = P-N Junctions Voltage
Since there is no voltage difference, the current can’t flow.
The only way for current to start flowing again is by external factors that can stimulate neutral atoms and cause them to lose electrons, breaking the equilibrium and creating a voltage difference.
Thus, generating a current flow (electricity).
And in the case of solar panels, our external factor is, yep you guessed it, sunlight.
Solar Cells Working Concept
Solar cells working concept is the photovoltaic effect, which is simply how light can knock electrons out of their atomic orbits, creating free electrons.
To understand how light can knock out electrons, the first thing you need to know is that light isn’t just a wave. In fact, light is made of very small particles called photons.
When a photon hits an orbiting electron, the electron absorbs some of the photon’s energy.
If this energy is big enough, the electron will gain enough power to break free from the nucleus magnetic force, resulting in a free electron.
In other words, if Absorbed Energy > Nucleus Electromagnetic Force, the electron will break free from the atom.
For simplicity’s sake, think of the photon as a tennis ball and the electron as a bottle of water.
If the photon hit the electron with enough power (you through the tennis ball as hard as you can), the electron will break free from the nucleus force (the bottle will fall off the fence).
Now, what would happen if multiple photons hit multiple electrons with enough force?
Exactly!
We will have a big flow of electrons (current) that we can use to power our mobile homes.
How Do Solar Panels Work Step By Step
1. Light Hits The Panel
As we discussed earlier, sunlight, or any type of light really, is made of small particles called photons.
Each photon has its own wavelength and that’s what determines the color and the energy of the photon.
Solar cells can only use photons in a very narrow range of wavelengths: 350 to 1140 nm.
In other words, only photons with energy >= the bandgap energy (the nucleus’s magnetic force) can be used to generate electricity.
2. Current Is Generated
When a photon with the right wavelength hits an electron, the electron will have enough energy to break free from the atom and become a loose electron.
When an electron becomes loose, it also leaves behind a hole.
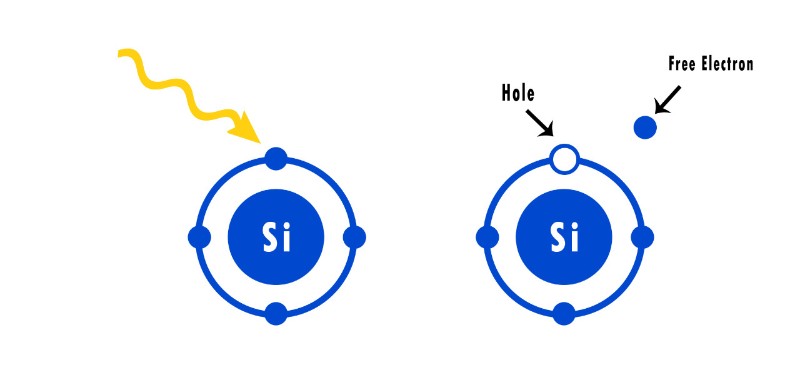
This breaks the P-N junction’s equilibrium, resulting in a flow of electrons and holes.
And the more photons hit the panel, the more free electrons we get, and the more electricity is generated.
3. Use The Electricity To Power The World
The final step would be collecting this energy.
When the photons separate the electrons from their atoms, it breaks the P-N junction equilibrium.
As a result, the holes will start attracting electrons.
And for electrons to reach the holes, they must go from the n-type material to the p-type through the depletion region, which has quite big resistance.
Now, we need these electrons to power our devices right?
So what we do is that we control their movement by providing an easier pathway with less resistance for electrons to reach the holes.
Obviously, this path is our home’s main electric panel.
And since our pathway has way less resistance than the depletion area, the electrons will flow through it, powering our appliances and recharging our batteries.
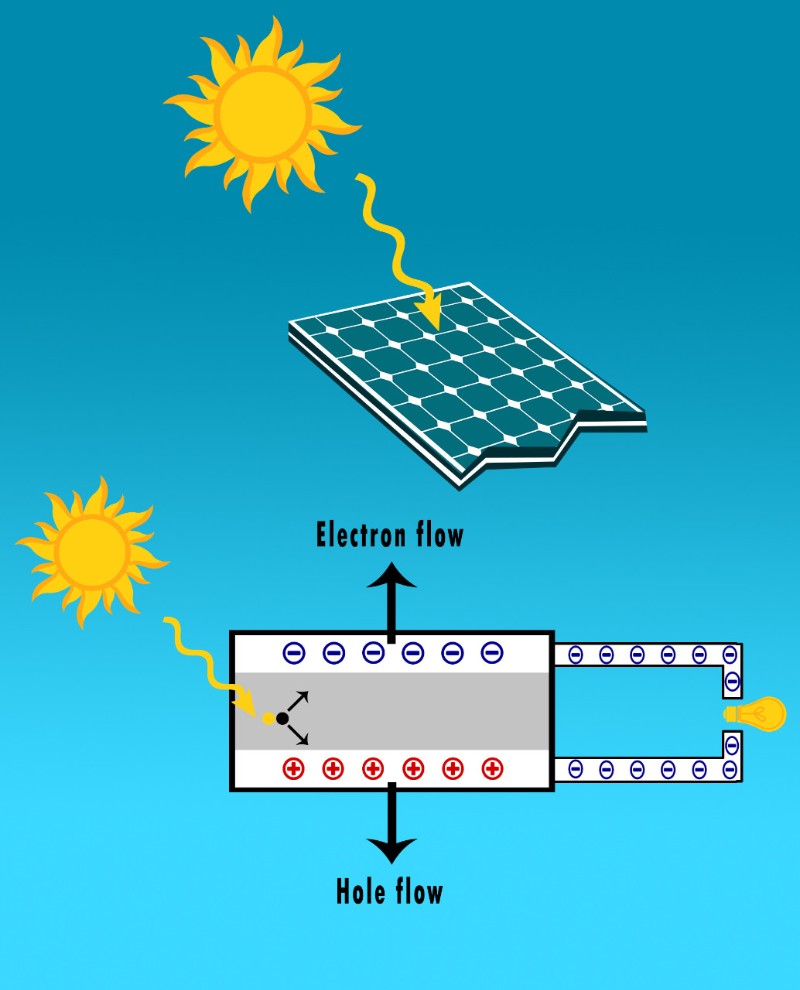
And that’s exactly how solar panels work.
They simply utilize the fact that light can knock out electrons of their orbit, so they amplify this effect by using doped semiconductors and low resistance wires to transfer this energy.
Last Words
As promised, in this article we showed you exactly how do solar panels work in as much detail as possible.
In the end, we really hope you enjoyed this article as much as we did.
Do you have any questions about solar cells?
If so, please share your thoughts with us in the comments section below.







Hi there to all, for the reason that I am genuinely keen of reading this website’s post to be updated on a regular basis. It carries pleasant stuff.